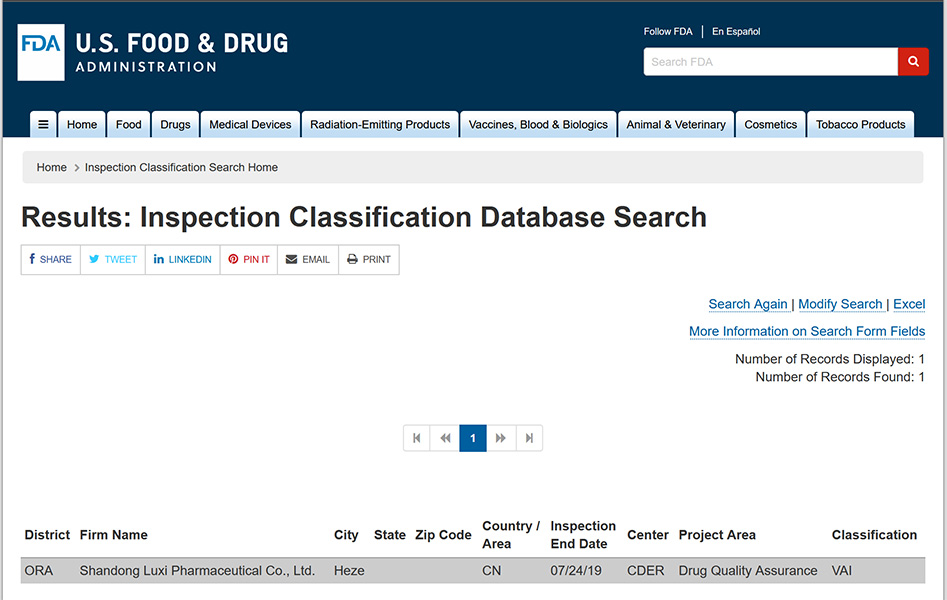
Bioengineering Division
Bioengineering Division undertakes the production of biological fermented API products of the company. The existing 661 production workshop was completed on April 20, 2013. The workshop is divided into two floors, the first floor has a construction area of 2290.28m2, the second floor has a construction area of 1017.68m2 and the total construction area is 3307.96m2. The clean area of 500m2 is divided into D clean area and C clean area. In June 2014, the Bioengineering Division obtained the API certificate for export to EU (Polymyxin B Sulphate); In March 2017, the API (polymyxin B sulfate) was registered in the US DMF file, file number: 031587; In September 2019, the API (Polymyxin B Sulfate) passed the FDA on-site audit; On December 16, 2020, the API (Polymyxin B Sulfate) has been re-reviewed and obtained the API Export Documents to the European Union. There are four ferferers of 10 tons, with an annual output of 2,000 kilograms and an annual output value of more than 40 million yuan. The API (Polymyxin B Sulfate) has been exported to Europe, America, Southeast Asia, Japan, India and other ranges and countries.